Difference Between Solution And Mixture
Mixture and solution are the basic concepts that must non be dislocated by the students. A mixture in simple words is a combination of usually 2 or more substances. The substances hither, are non chemically combined and their properties remain the same. Since it is combined physically, the identities of individual substances are retained. Mixtures can be either heterogeneous or homogeneous, depending upon the uniformity of components. The solution, on the other paw, is completely different from a mixture. The solution is a blazon of mixture, wherein the substances are dissolved. In other words, the solution is a homogeneous mixture containing i or more solutes in a solvent. An instance of a solution tin can be carbohydrate syrup. Thus, a solution is a type of mixture, but a mixture may or may not exist a solution. Read further to sympathize better the departure between the 2.
Cardinal takeaways: Mixture, Properties, Types, Solution, Characteristics, Examples, Difference between mixture and solution.
Read more: Carbonyl Compounds
What is Mixture?
[Click Hither for Sample Questions]
The combination of two or more substances, without bringing in whatsoever chemic change is known as a mixture in chemistry. When the substances in a mixture combine together, they practice neither lose their individual properties nor alter their chemical bondings. They usually result in a production of a chemic combining of substances.
Some of the examples of mixtures include; a mixture of water and salt, like seawater, a mixture of gases similar oxygen, carbon dioxide, nitrogen, carbon monoxide, etc forming air, a mixture of unlike types of colored dyes forming ink.
Backdrop of Mixtures
The main properties of mixtures are given below:
- The original backdrop of a mixture exercise not alter.
- The proportion of ratio of the constituents can vary.
- The constituents of a mixture tin be separated with the aid of mechanical means.
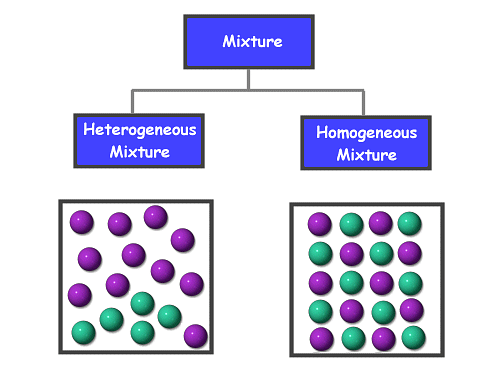
Types of Mixtures
Mixtures are of ii types:
Homogeneous mixture
It is the one wherein 2 compounds have similar characteristics are mixed with each other, such that they are compatible throughout. For case, table salt and water.
Heterogeneous mixture
Information technology is the one wherein 2 different types of compounds with dissimilar properties are mixed with one another. For example, salt and sand are mixed together.
Read too:
What is Solution?
[Click Here for Sample Questions]
The solution is a homogeneous mixture, wherein the size of particles is not more than 1nm. Information technology consists of ii substances, namely, solute and solvent. The solution can exist in both liquid gaseous forms.

The solute is a substance that is dissolved in a solvent and is much less in quantity than a solvent. A solvent, on the other paw, is an aqueous component in which a solute is dissolved. It'south quantity is more than the solute. Some of the common examples of solutions include; air, saccharide syrup, etc.
Characteristics of Solutions
- A solution is a homogeneous mixture.
- The particles of a solution are less than 1nm in size.
- A solution as a mixture is stable in nature.
- The constituents of a solution cannot be separated with the help of mechanical methods.
Types of Solutions
Solutions take been divided into 9 main categories:
- Solid-solid
- Solid-liquid
- Solid-gas
- Liquid-liquid
- Liquid-solid
- Liquid-gas
- Gas-liquid
- Gas-solid
- Gas-gas
Read more than:
Difference betwixt Mixture and Solution
[Click Here for Sample Questions]
The departure between solution and mixture is mentioned in the table beneath.
| Mixture | Solution |
|---|---|
| Substances are combined physically in a mixture. | The solutes become dissolved in the solvent and tin can be separated using mechanical methods. |
| In a mixture, substances are just mixed and are not completely dissolved. | In a solution, substances are dissolved completely and cannot be filtered out. |
| The chemical properties of each substance are retained. | Chemic properties change. |
| The number of substances in a mixture tin can vary equally they exercise not accept a fixed ratio. | A solution ordinarily has a fixed ratio of the number of substances. |
| The mixture can be either heterogeneous or homogeneous. | A solution is a homogeneous mixture. |
Things to Remember
- Mixture is the combination of ii or more substances, without bringing in any chemical change.
- A mixture tin exist heterogeneous or homogeneous.
- Solution is a type of homogeneous mixture, wherein the substances are dissolved chemically.
- In a mixture, the chemical properties of a substance are retained, while, they change in a solution.
Read more than: High-Density Polyethylene construction
Sample Questions
Ques. What is a mixture? Give 2 examples. (2 marks)
Ans: Mixture is the combination of 2 or more than substances, that are formed without bringing in any chemical modify. The substances hither, are non chemically combined and their backdrop remain the same. A mixture of water and salt, like seawater, or a mixture of gases in the air are examples of a mixture.
Ques. What is a solution? Give two characteristics of the same. (two marks)
Ans: Solution is a type of homogeneous mixture, wherein the substances are dissolved using chemical methods. In a solution, substances are dissolved completely and cannot be filtered out. A solution is a homogeneous mixture. The particles of a solution are less than 1nm in size.
Ques. What is the major difference between a mixture and a solution? (two marks)
Ans: • Substances in a mixture are combined physically, while those in a solution are dissolved using mechanical methods.
- The chemical properties of substances in a mixture are retained, while those of a solution practice change.
- The amount of substances in a mixture is not in a fixed ratio, while in a solution, the substances are in a stock-still ratio.
Ques. Is sugar syrup a mixture or a solution? (2 marks)
Ans: Sugar syrup is a type of solution. It is a liquid-solid mixture, wherein the saccharide is diluted in water. Here, sugar acts as a solute, whereas water acts as a solvent.
Ques. What is a heterogeneous mixture? (2 marks)
Ans: A heterogeneous mixture is a mixture having non-uniform composition. Hither, two different types of compounds with different properties are mixed with each other. The limerick of mixtures of sodium chloride and atomic number 26 fillings, oil, and water are some of the examples of a heterogeneous mixture.
Ques. Which of the following substances autumn in the category of mixtures. (2 marks)
(a) Sodium
(b) Soil
(c) Saccharide solution
(d) Silver
(e) Calcium carbonate
(f) Tin
(thousand) Silicon
(h) Coal
Ans: The following substances can exist classified as mixtures:
Soil
Saccharide solution
Coal
Ques. Allocate each of the following as heterogeneous or homogeneous mixture. Soda water, forest, air, soil, vinegar, filtered tea(ii marks)
Ans: Homogeneous mixtures: Soda water, air, vinegar
Heterogeneous mixtures: Wood, soil, filtered tea
Ques. Identify which of the post-obit mixtures are solutions: (2 marks)
(a) Soil
(b) Bounding main water
(c) Air
(d) Coal
(e) Soda water
Ans: The following mixtures are solutions:
Sea water
Soda water
Air
Chemistry Related Links:
Difference Between Solution And Mixture,
Source: https://collegedunia.com/exams/difference-between-mixture-and-solution-science-articleid-5271
Posted by: judgemolon1941.blogspot.com


0 Response to "Difference Between Solution And Mixture"
Post a Comment